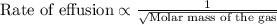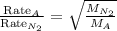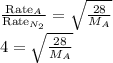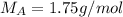A sample of N2 gas is contaminated with a gas (A) of unknown molar mass. The partial pressure of each gas is known to be 200 tore at 25 degrees Celsius. The gas are allowed to Effuse through a pinhole, and it is found that has A escapes at 4 times the rate of N2. The molar mass of gas A is:
A. 1.75 g/mol
B. 448 g/mol
C. 112 g/mol
D. 7.01 g/mol
E. None of these

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, Slycooper5959
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 22.06.2019 19:00, montgomerykarloxc24x
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 09:00, alisonlebron15
Are the results of a thoroughly tested hypothesis?
Answers: 2
Do you know the correct answer?
A sample of N2 gas is contaminated with a gas (A) of unknown molar mass. The partial pressure of eac...
Questions in other subjects:

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 08:01

Mathematics, 18.09.2020 09:01

Mathematics, 18.09.2020 09:01

Biology, 18.09.2020 09:01

Mathematics, 18.09.2020 09:01

Mathematics, 18.09.2020 09:01

Mathematics, 18.09.2020 09:01

Mathematics, 18.09.2020 09:01

Mathematics, 18.09.2020 09:01