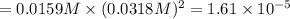PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)

Chemistry, 15.04.2020 00:52, genyjoannerubiera
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Suppose you add 0.2307 g of PbCl2 (s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of Pb2+ (aq) is 0.0159 M and the concentration of Cl− (aq) is 0.0318 M.
What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
Do you know the correct answer?
Consider the dissolution equation of lead(II) chloride.
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
PbCl2 (s) ⇆ Pb2+ (aq) + 2Cl− (aq)
Questions in other subjects:

Social Studies, 21.07.2019 03:31


Biology, 21.07.2019 03:31

Biology, 21.07.2019 03:31


Biology, 21.07.2019 03:31





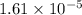 .
.![[Pb^{2+}]=0.0159 M](/tpl/images/0600/3825/89ec5.png)
![[Cl^-]=0.0318 M](/tpl/images/0600/3825/75929.png)
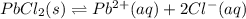
![K_{sp}=[Pb^{2+}][Cl^-]^2](/tpl/images/0600/3825/7fd11.png)