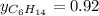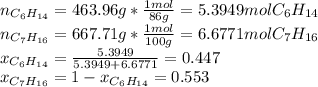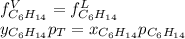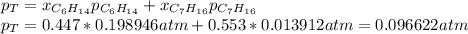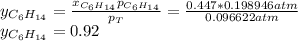Chemistry, 15.04.2020 00:51, malachilaurenc
Two linear hydrocarbons, Hexane (C6H14) and Heptane (C7H16), form pretty much an ideal solution at any composition. A solution is made at 25°C that contains 463.96 g of Hexane in 667.71 g Heptane: Characterise the vapour above this solution, and answer, What is the mole fraction of Hexane in the vapour?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, emilyswinge4421
Listenbase your answer to the question on the information below. nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Do you know the correct answer?
Two linear hydrocarbons, Hexane (C6H14) and Heptane (C7H16), form pretty much an ideal solution at a...
Questions in other subjects:


Mathematics, 24.03.2020 06:28



Physics, 24.03.2020 06:28

Mathematics, 24.03.2020 06:29

Mathematics, 24.03.2020 06:29


Mathematics, 24.03.2020 06:29