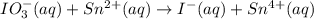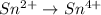Given the partial equation:
IO³⁻ (aq) + Sn²⁺ (aq) → I⁻ (aq) + Sn⁴⁺ (aq),
balance the reaction in acidic solution using the half-reaction method and fill in the coefficients.
The missing blanks represent H2O, H+, or OH-, as required to balance the reaction. Enter the coefficients as integers, using the lowest whole numbers. If the coefficient for something is "1", make sure to type that in and not leave it blank. Enter only the coefficients.
IO³⁻ (aq) + Sn²⁺ (aq) + → I⁻ (aq) + Sn⁴⁺ (aq) +

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1


Chemistry, 23.06.2019 15:00, maariaaa10
An isotope undergoes radioactive decay by emitting radiation that has no mass. what other characteristic does the radiation have?
Answers: 3
Do you know the correct answer?
Given the partial equation:
IO³⁻ (aq) + Sn²⁺ (aq) → I⁻ (aq) + Sn⁴⁺ (aq),
balance the r...
IO³⁻ (aq) + Sn²⁺ (aq) → I⁻ (aq) + Sn⁴⁺ (aq),
balance the r...
Questions in other subjects:


Biology, 11.07.2019 22:30

Biology, 11.07.2019 22:30


Mathematics, 11.07.2019 22:30

Biology, 11.07.2019 22:30

Biology, 11.07.2019 22:30



Biology, 11.07.2019 22:30



 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.