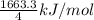Chemistry, 15.04.2020 02:05, melissakm77
Given that Δ H ∘ f [ H ( g ) ] = 218.0 kJ ⋅ mol − 1 Δ H ∘ f [ C ( g ) ] = 716.7 kJ ⋅ mol − 1 Δ H ∘ f [ CH 4 ( g ) ] = − 74.6 kJ ⋅ mol − 1 calculate the average molar bond enthalpy of the carbon‑hydrogen bond in a CH 4 molecule

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3



Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
Do you know the correct answer?
Given that Δ H ∘ f [ H ( g ) ] = 218.0 kJ ⋅ mol − 1 Δ H ∘ f [ C ( g ) ] = 716.7 kJ ⋅ mol − 1 Δ H ∘ f...
Questions in other subjects:

Computers and Technology, 12.12.2020 23:10





Advanced Placement (AP), 12.12.2020 23:10

Social Studies, 12.12.2020 23:10

History, 12.12.2020 23:10


Health, 12.12.2020 23:10


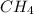 is 415.825 kJ/mol.
is 415.825 kJ/mol.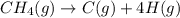
![\Delta H^{0}=[1mol\times \Delta H_{f}^{0}(C)_{g}]+[4mol\times \Delta H_{f}^{0}(H)_{g}]-[1mol\times \Delta H_{f}^{0}(CH_{4})_{g}]](/tpl/images/0600/7413/0c0db.png)
![[1mol\times 716.7\frac{kJ}{mol}]+[4mol\times 218.0\frac{kJ}{mol}]-[1mol\times -74.6\frac{kJ}{mol}]=1663.3kJ](/tpl/images/0600/7413/961ee.png)