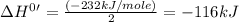Chemistry, 15.04.2020 02:09, claudia122752
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphite) + 2 H2(g) + O2(g) ΔH° = 232 kJ What is the standard enthalpy change for this reaction at 298 K? C(s, graphite) + H2(g) + 1/2 O2(g) H2CO(g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 04:00, eborkins
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
Do you know the correct answer?
The standard enthalpy change for the following reaction is 232 kJ at 298 K. 2 H2CO(g) 2 C(s, graphit...
Questions in other subjects:






History, 03.11.2020 18:30

Mathematics, 03.11.2020 18:30

History, 03.11.2020 18:30





 for the following reaction i.e,
for the following reaction i.e,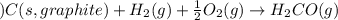

 for the reaction will be:
for the reaction will be: