

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 23.06.2019 01:30, Nathaliasmiles
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Do you know the correct answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + N2(g) → 2L...
Questions in other subjects:

Mathematics, 08.10.2021 01:20

English, 08.10.2021 01:20

Mathematics, 08.10.2021 01:20



Social Studies, 08.10.2021 01:20





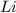 are needed to produce 0.45 mol of
are needed to produce 0.45 mol of 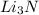
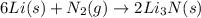
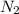 is the excess reagent.
is the excess reagent.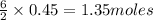 of
of 



