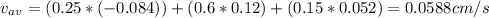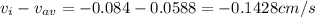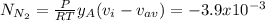A gaseous mixture at 265K and 1.0 atm contains 25 O2 60 N2 and 15 CO2 mole basis The velocities of the components are 0.084 cm s O2 0.120 cm s N2 and 0.052 cm s CO2 Find the N2 diffusion velocity relative to the mole average velocity and the molar diffusional flux of N2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, pennygillbert
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1


Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 08:00, vetterk1400
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
Do you know the correct answer?
A gaseous mixture at 265K and 1.0 atm contains 25 O2 60 N2 and 15 CO2 mole basis The velocities of t...
Questions in other subjects:


Biology, 25.03.2020 06:54

Mathematics, 25.03.2020 06:54



Mathematics, 25.03.2020 06:55


History, 25.03.2020 06:55