
Chemistry, 14.04.2020 21:42, jybuccaneers2022
Diiodine pentoxide (I2O5) is used in respirators to remove carbon monoxide (CO) from air according to the chemical equation: I2O5 (s) + 5 CO (g) --> I2 (s) + 5 CO2 (g) What mass of carbon monoxide could be removed from air by a respirator that contains 15.9 g of diiodine pentoxide? The molar mass of I2O5 is 333.8 g / mol.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
Do you know the correct answer?
Diiodine pentoxide (I2O5) is used in respirators to remove carbon monoxide (CO) from air according t...
Questions in other subjects:


Mathematics, 02.12.2020 05:00


Mathematics, 02.12.2020 05:00

Geography, 02.12.2020 05:00

Business, 02.12.2020 05:00

Mathematics, 02.12.2020 05:00

English, 02.12.2020 05:00

Mathematics, 02.12.2020 05:00

Mathematics, 02.12.2020 05:00


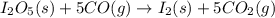
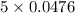 of CO from air
of CO from air



