
Chemistry, 14.04.2020 21:12, larapoghosyan91
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-calming agent. What is the hydronium ion concentration (in M) in a 0.737 M solution of HC5H9O2? (Assume Kw = 1.01 ✕ 10−14.)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3


Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
Do you know the correct answer?
Valeric acid, HC5H9O2 (Ka = 1.5 ✕ 10−5), is used in the manufacture of magnesium valerate, a nerve-c...
Questions in other subjects:


Mathematics, 15.07.2019 13:30




Mathematics, 15.07.2019 13:30



Social Studies, 15.07.2019 13:30

Mathematics, 15.07.2019 13:30


![[H^+]=0.00332M](/tpl/images/0599/2796/eac47.png)
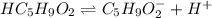
![Ka=\frac{[H^+][C_5H_9O_2^-]}{[HC_5H_9O_2]}](/tpl/images/0599/2796/6b49e.png)
 due to dissociation, it becomes:
due to dissociation, it becomes: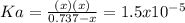
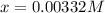
![[H^+]=x=0.00332M](/tpl/images/0599/2796/13d16.png)




