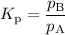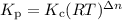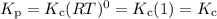Chemistry, 14.04.2020 21:01, damari9288
Select the statements below that are TRUE. Kc has units of molarity and Kp has units of pressure Kp uses the same equation as Kc, but with pressure instead of concentration If there is a liquid in a reaction, you use the vapor press for that entry in the Kp equation. Kc and Kp are the same thing as the k (rate constant) that you learned about before. Kc is equal to Kp when the number of moles of gas (n) doesn't change. Solids and liquid are not included in the Kc and Kp equations because they have an activity equal to 1.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 00:00, savyblue1724707
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
Do you know the correct answer?
Select the statements below that are TRUE. Kc has units of molarity and Kp has units of pressure Kp...
Questions in other subjects:

Biology, 17.12.2020 19:00






Mathematics, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00

Law, 17.12.2020 19:00


![K_{\text{c}} = \dfrac{\text{[B]}}{\text{[A]}}](/tpl/images/0599/2268/ba231.png)