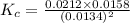Chemistry, 14.04.2020 20:01, dontworry48
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles of CH4, and 0.240 moles of CCl4 are at equilibrium in a 15.2 L container at 477 K, the value of the equilibrium constant, Kc, is

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kathleensumter4913
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 22.06.2019 00:40, draveon353
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1
Do you know the correct answer?
Consider the following reaction: 2CH2Cl2(g) CH4(g) CCl4(g) If 0.203 moles of CH2Cl2(g), 0.323 moles...
Questions in other subjects:

Mathematics, 11.09.2021 01:50


Mathematics, 11.09.2021 01:50





Mathematics, 11.09.2021 01:50



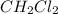 at equilibrium= 0.203 mole
at equilibrium= 0.203 mole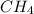 at equilibrium = 0.323 mole
at equilibrium = 0.323 mole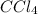 at equilibrium = 0.240mole
at equilibrium = 0.240mole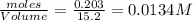
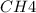 =
= 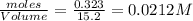
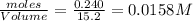
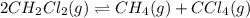
![K_c=\frac{[CH_4]\times [CCl_4]}{[CH_2Cl_2]^2}](/tpl/images/0599/0518/28fa5.png)