
Chemistry, 14.04.2020 19:35, baby092000
For the reaction 2Co3+(aq)+2Cl−(aq)→2Co2+(aq)+Cl2(g) . E∘=0.483 V2Co3+(aq)+2Cl−(aq)→2Co2+(aq)+Cl2(g ). E∘=0.483 V what is the cell potential at 25 ∘C∘C if the concentrations are [Co3+]=[Co3+]= 0.324 MM , [Co2+]=[Co2+]= 0.158 MM , and [Cl−]=[Cl−]= 0.384 MM , and the pressure of Cl2Cl2 is PCl2=PCl2= 5.80 atmatm ?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Do you know the correct answer?
For the reaction 2Co3+(aq)+2Cl−(aq)→2Co2+(aq)+Cl2(g) . E∘=0.483 V2Co3+(aq)+2Cl−(aq)→2Co2+(aq)+Cl2(g...
Questions in other subjects:


Social Studies, 05.06.2021 01:30

Mathematics, 05.06.2021 01:30

Geography, 05.06.2021 01:30





English, 05.06.2021 01:30


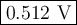
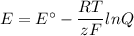
![Q = \dfrac{\text{[Cl}^{-}]^{2}[\text{Co}^{3+}]^{2}}{p_{\text{Cl}_{2}}^{2}\text{[Co}^{3+}]^{2}} = \dfrac{0.384^{2} \times 0.324^{2}}{5.80 \times 0.158^{2}} =0.1069\\\\E = 0.483 - \left (\dfrac{8.314 \times 298.15 }{2 \times 96485}\right ) \ln(0.1069)\\\\=0.483 -0.01285 \times (-2.236) = 0.483 + 0.02872 = \textbf{0.512 V}\\\text{The cell potential is } \large\boxed{\textbf{0.512 V}}](/tpl/images/0598/9253/e9761.png)





