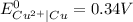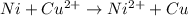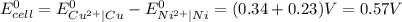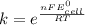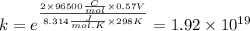Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²...

Chemistry, 14.04.2020 18:32, lovemichelle638
Use standard reduction potentials to calculate the equilibrium constant for the reaction:
Cu²⁺ (aq) + Ni(s) → Cu(s) + Ni²⁺ (aq)
Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kaylaamberd
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 22.04.2021 02:40

French, 22.04.2021 02:40

Mathematics, 22.04.2021 02:40


Mathematics, 22.04.2021 02:40



World Languages, 22.04.2021 02:40

Mathematics, 22.04.2021 02:40



 ;
; 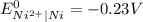
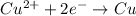 ;
;