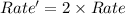Chemistry, 14.04.2020 18:03, mayamcmillan11
Consider the reaction NaCH3COO (sodium acetate in acetic acid) with tert-butyl bromide (2-bromo-2-methylpropane). If the concentration of both the nucleophile/base and the substrate are doubled, what happens to the rate of reaction?
a. it doubles
b. it quadruples
c. new rate = 1/2 of original rate
d. it does not change
e. new rate = 1/4 of original rate

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 07:20, prettydoll19
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
Do you know the correct answer?
Consider the reaction NaCH3COO (sodium acetate in acetic acid) with tert-butyl bromide (2-bromo-2-me...
Questions in other subjects:

Mathematics, 31.08.2019 14:30

Computers and Technology, 31.08.2019 14:30


Social Studies, 31.08.2019 14:30

Mathematics, 31.08.2019 14:30


Biology, 31.08.2019 14:30



English, 31.08.2019 14:30


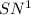 reaction and thus rate depends only on the concentration of tert-butyl bromide.
reaction and thus rate depends only on the concentration of tert-butyl bromide.![Rate=k[tertbutylbromide]^1](/tpl/images/0598/5017/a90bc.png) [base]^0
[base]^0![Rate'=k[2\times tertbutylbromide]^1[2\times base]^0}](/tpl/images/0598/5017/d2259.png)
![Rate'=k2^1[tertbutylbromide]^1[base]^0](/tpl/images/0598/5017/cfc9c.png) (2)
(2)