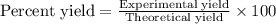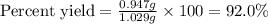Chemistry, 14.04.2020 18:07, Gabriella0000
A 1.103 g sample of sodium fluoride is dissolved in water, and then a precipitate of calcium fluoride is produced by adding a calcium nitrate solution. If the dried calcium fluoride precipitate has a mass of 0.947 g, what is the percent yield?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3


Chemistry, 23.06.2019 06:20, ratpizza
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 09:00, msladycie8831
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1
Do you know the correct answer?
A 1.103 g sample of sodium fluoride is dissolved in water, and then a precipitate of calcium fluorid...
Questions in other subjects:


Mathematics, 10.11.2021 21:30

Mathematics, 10.11.2021 21:30

French, 10.11.2021 21:30

Chemistry, 10.11.2021 21:30




Mathematics, 10.11.2021 21:30


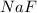 = 1.103 g
= 1.103 g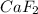 = 78 g/mol
= 78 g/mol

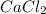

 mole of
mole of