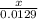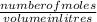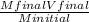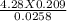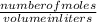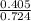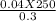Chemistry, 14.04.2020 07:44, aide1234564
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (aq) -->
2. How many L of a 0.209 M KI solution is needed to completely react with 2.43 g of Cu(NO3)2 according to the balanced chemical reaction:
2Cu(NO₃)₂(aq) + 4KI(aq) → 2CuI(aq) + I₂(s) + 4KNO₃(aq)
3.What volume in L of a 0.724 M Nal solution contains0.405 mol of Nal?
4. How many mL of 0.300 M NaF would be required to make a 0.0400 M solution of NaF when diluted to 250.0 mL with water?

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, ashtonviceoxd21i
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b. colder climates near the equator c. large waves on the cost of europe d. warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
Do you know the correct answer?
1.Complete the balanced neutralization equation for the reaction below:
H2SO4 (aq) + Sr(OH)2 (...
H2SO4 (aq) + Sr(OH)2 (...
Questions in other subjects:


Mathematics, 13.03.2021 03:40

Mathematics, 13.03.2021 03:40

History, 13.03.2021 03:40




Advanced Placement (AP), 13.03.2021 03:40



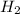 S
S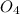 + Sr(OH)
+ Sr(OH) ⇒ SrSO4 + 2 H2O is the balanced reaction.
⇒ SrSO4 + 2 H2O is the balanced reaction.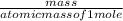
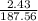
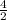 =
=