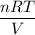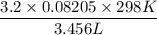HEN
A 45.2 g sample of Nitrogen gas, N2, has a volume of 3,456mL and a
temperature of 25...

Chemistry, 14.04.2020 06:12, faithlopez209
HEN
A 45.2 g sample of Nitrogen gas, N2, has a volume of 3,456mL and a
temperature of 25.0 °C. What is the pressure of the gas? *
F
O 1160 kPa
O 1.15 kPa
O 11.4 kPa
SES
EBRER
96.8 kPa

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, ramireznaidelyn
4nh3+5o2--> 4no+6h20what is the total number of moles of h2o produced when 12 mole of nh3 is completely consumed?
Answers: 3


Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
Do you know the correct answer?
Questions in other subjects:


English, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30

Mathematics, 13.07.2019 05:30


Social Studies, 13.07.2019 05:30


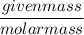 =
= 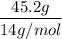 = 3.2 mol
= 3.2 mol