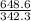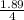Chemistry, 13.04.2020 22:49, 20warriorsoul14
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of the solution?
The chemical formula for sucrose is C12H22O11 , and the molar mass is 342.3 g/mol. mol/L

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
Do you know the correct answer?
If 684.6g of sucrose (table sugar) are dissolved in 4 liters of water, what would be the molarity of...
Questions in other subjects:

Geography, 06.10.2019 05:00

Physics, 06.10.2019 05:00


English, 06.10.2019 05:00





Physics, 06.10.2019 05:00

Mathematics, 06.10.2019 05:00


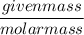
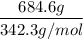
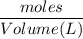

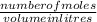 equation 1
equation 1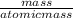 equation 2
equation 2