6. which of the following statements is true?
A. in an exothermic reaction, the energy of the...

6. which of the following statements is true?
A. in an exothermic reaction, the energy of the products is the same as the energy of the reactants.
B. in an endothermic reaction, the energy of the products is the same as the energy of the reactants.
C. in an exothermic reaction, the energy of the products is less than the energy of the reactance.
D. in an endothermic reaction, the energy of the products is less than the energy of the reactants.
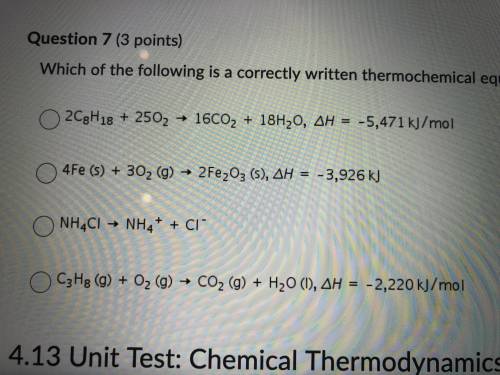
7. which of the following is correctly written thermochemical equation?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1


Chemistry, 23.06.2019 00:20, HernanJe6
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Do you know the correct answer?
Questions in other subjects:


Mathematics, 30.03.2021 04:30

Mathematics, 30.03.2021 04:30

Biology, 30.03.2021 04:30




Computers and Technology, 30.03.2021 04:30

Mathematics, 30.03.2021 04:30







