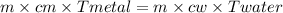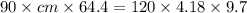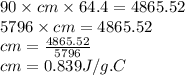specific heat of metal piece is 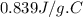 .
.
Explanation:
As it is problem of convective heat and mass transfer, concept of energy balance can be used. The phenomenon occur here is natural. As temperature of metal is higher than that of water, so flow of temperature is from higher temperature to lower temperature, heat will flow from metal piece to water.
Data Given are as follows:
mass of piece of metal = 90 g
mass of water = 120 g
specific heat of water = 4.18 J/g.°C
specific heat of metal = ?
T metal,i = 98.6°C ...Initial temperature of metal piece
T water,i = 24.3°C ...Initial temperature of water
T metal ,f = 34°C ...Initial temperature of metal piece
T water,f = 34°C ...Initial temperature of water
ΔT water = T water,f - T water,i = 34°C - 24.3°C
ΔT metal = T metal,i - T metal,f = 98.6°C - 34°C
It is easily understood that whatever amount of heat is liberated by metal piece absorbed by water. Heat transfer is limited to thermal equilibrium between water and metal piece. Final condition is such that temperature of water will rise upto 34°C and temperature of metal will drop to 34°C.
Heat lost by metal = Heat gained by water
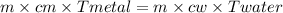
Putting values in above equation,
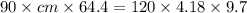
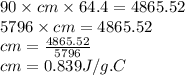
Therefore, specific heat of metal piece is 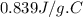 .
.
















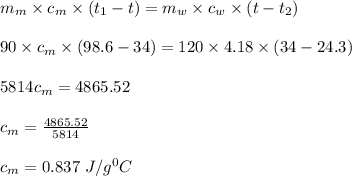
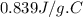 .
.