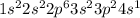Chemistry, 11.04.2020 04:03, browneyedbaby20
Which of the following electron configurations represents an excited state of the indicated atom? Group of answer choices
a. Na: 1s2 2s2 2p6 3s2 3p2 3s1
b. Ne: 1s2 2s2 2p6
c. N: 1s2 2s2 2p3
d. P: 1s2 2s2 2p6 3s2 3p2 4s1
e. He: 1s2

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mommatann
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 14:00, MathChic68
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
Which of the following electron configurations represents an excited state of the indicated atom? Gr...
Questions in other subjects:




Chemistry, 25.07.2019 13:40

Spanish, 25.07.2019 13:40


History, 25.07.2019 13:40