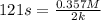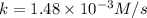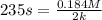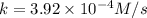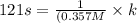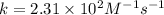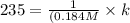Chemistry, 11.04.2020 03:07, reneebrown017
A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.357 M. The same reactant decomposes with a half-life of 235 s when its initial concentration is 0.184 M.
What is the value and unit of the rate constant for this reaction?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
Do you know the correct answer?
A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.357 M...
Questions in other subjects:


Engineering, 25.05.2021 19:40

World Languages, 25.05.2021 19:40





Mathematics, 25.05.2021 19:40


Chemistry, 25.05.2021 19:40


 and
and 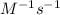 respectively.
respectively.![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0594/9562/b5b11.png)
![t_{1/2}=\frac{1}{[A_o]k}](/tpl/images/0594/9562/15d11.png)