

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, hdjsjfjruejchhehd
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
Do you know the correct answer?
Calculate the number of moles of oxygen gas that are required to react with 4.74 mol dodecane, C 10H...
Questions in other subjects:


Physics, 23.02.2021 21:10

History, 23.02.2021 21:10

Mathematics, 23.02.2021 21:10


History, 23.02.2021 21:10

Mathematics, 23.02.2021 21:10


Mathematics, 23.02.2021 21:10

Mathematics, 23.02.2021 21:10


 .
. 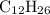 . (In an alkane molecule with
. (In an alkane molecule with 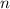 carbon atoms, there would be
carbon atoms, there would be 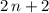 hydrogen atoms.)
hydrogen atoms.) .
. (the number of moles of dodecane in the reaction) and is asking for
(the number of moles of dodecane in the reaction) and is asking for 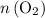 (the number of moles of oxygen in this reaction.) It would be helpful if there is a ratio
(the number of moles of oxygen in this reaction.) It would be helpful if there is a ratio 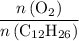 for directly converting
for directly converting 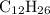 is
is  , andthe coefficient of
, andthe coefficient of  is
is  .
. of
of 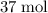 . Note that in this example, there's nothing special about the quantity of one mole. It is possible to scale the quantities of both reactants, and this ratio would still hold for this reaction. Thus,
. Note that in this example, there's nothing special about the quantity of one mole. It is possible to scale the quantities of both reactants, and this ratio would still hold for this reaction. Thus, 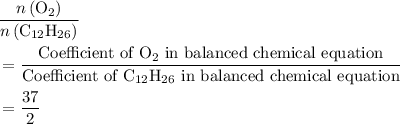 .
.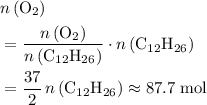 .
.



