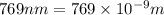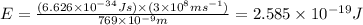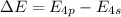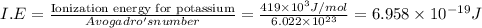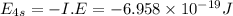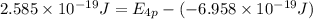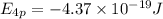Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:00, winstonbendariovvygn
Diffraction is when light is bent around obstructions. which of the these observation about clouds would indicate diffraction? a) after rain storms, you can sometimes see rainbows. b) clouds are white or gray and cannot be seen through. c) on a cloudy day, the temperature tends to be cooler than a sunny day. d) the edges of dark clouds appear lighter. this
Answers: 3

Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
Do you know the correct answer?
The ionization energy for potassium 419 kj/mol. the wavelength of light emitted when an excited k at...
Questions in other subjects:


English, 20.06.2021 23:00

Chemistry, 20.06.2021 23:00





Chemistry, 20.06.2021 23:00

Mathematics, 20.06.2021 23:00

Mathematics, 20.06.2021 23:00


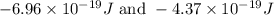
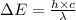
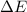 = change in energy
= change in energy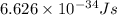
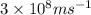
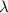 =
=