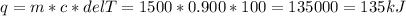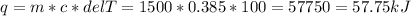Chemistry, 09.04.2020 23:37, walkerobrien5
If you have a 1500 g aluminum pot, how much heat energy is needed to raise its temperature by 100°C?
If you have a 1500 g copper pot, how much heat energy is needed to raise its temperature by 100°C?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 21:30, kawaiiblurainbow
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
Do you know the correct answer?
If you have a 1500 g aluminum pot, how much heat energy is needed to raise its temperature by 100°C?...
Questions in other subjects:





Computers and Technology, 18.08.2021 19:20

Chemistry, 18.08.2021 19:20

English, 18.08.2021 19:20


Arts, 18.08.2021 19:20