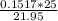Chemistry, 09.04.2020 04:37, Bearboy5957
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indicator are added to an erlenmeyer flask, and the titration experiment is carried out. if the initial buret reading was 0.55 ml, and the buret reading at the endpoint was 22.50 ml, what is the molarity of the unknown acid, ha?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 22.06.2019 18:00, jessicannoh5965
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
Do you know the correct answer?
A buret is filled with 0.1517 m naoh (aq). 25.0 ml of an unknown acid ha (aq) and two drops of indic...
Questions in other subjects:

Mathematics, 05.11.2020 01:00

English, 05.11.2020 01:00


English, 05.11.2020 01:00



English, 05.11.2020 01:00


Spanish, 05.11.2020 01:00


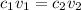 where 1 and 2 are the values for acid and base respectively.
where 1 and 2 are the values for acid and base respectively.