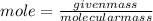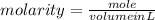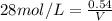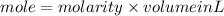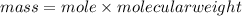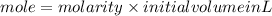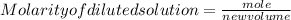1. What volume in mL of 28.0M H2SO4 is needed to contain 53.0g of H2SO4?
2. How many gra...

1. What volume in mL of 28.0M H2SO4 is needed to contain 53.0g of H2SO4?
2. How many grams of calcium hydroxide Ca(OH)2 are needed to make 532.0 mL of a 1.90M solution?
3. If 12 mL of water are added to 124mL of a 0.505M K2SO4 solution, what will the molarity of the diluted solution be?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, officialgraciela67
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Social Studies, 18.12.2020 22:50

Mathematics, 18.12.2020 22:50




Mathematics, 18.12.2020 22:50

Mathematics, 18.12.2020 22:50


Mathematics, 18.12.2020 22:50