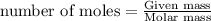Chemistry, 24.10.2019 21:43, valenciafaithtorres
The mass and volume of each sample differ from the mass and volume of the other samples. is it possible for each sample to contain 1 mol of each substance?
a) no, because they have different masses.
b) no, because they have different volumes.
c) yes, because the number of moles is not dependent on the mass or the volume.
d) yes, because the number of moles is only dependent on the mass per unit volume.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
Do you know the correct answer?
The mass and volume of each sample differ from the mass and volume of the other samples. is it possi...
Questions in other subjects:




Mathematics, 17.10.2020 16:01

Mathematics, 17.10.2020 16:01

Biology, 17.10.2020 16:01

Social Studies, 17.10.2020 16:01