
Chemistry, 08.04.2020 22:29, ayoismeisalex
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number of moles of SrCl2 is consumed when 54 g of ZnCl2 is produced?
a) 0.16 b) 0.3 c) 0.79 d) 1.58 e) 0.4

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
Do you know the correct answer?
Given the balanced equation:
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
ZnSO4 + SrCl2 > SrSO4 + ZnCl2
What number...
Questions in other subjects:

Mathematics, 02.10.2020 19:01



English, 02.10.2020 19:01



Mathematics, 02.10.2020 19:01



Mathematics, 02.10.2020 19:01


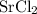 . consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.
. consumed by 54 grams of zinc chloride has been 0.4 moles. Thus the correct option is e.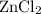 consumes 1 mole of
consumes 1 mole of 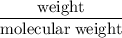
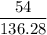 mol
mol



