
Use the thermochemical equations shown below to determine the enthalpy for the reaction: COCl2(g) + H2O(l) --> CH2Cl2(l) + O2(g) ½H2(g) + ½Cl2(g) --> HCl(g) ΔH=-46kJ H2O(g) + Cl2(g) --> 2HCl(g) + ½O2(g) ΔH=-21KJ CH2Cl2(l) + H2(g) + O2(g) --> COCl2(g) + 2H2O(l) ΔH = 80.5 kJ

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1

Do you know the correct answer?
Use the thermochemical equations shown below to determine the enthalpy for the reaction: COCl2(g) +...
Questions in other subjects:


English, 01.03.2021 18:50

Chemistry, 01.03.2021 18:50

Mathematics, 01.03.2021 18:50

Mathematics, 01.03.2021 18:50



Mathematics, 01.03.2021 18:50

Mathematics, 01.03.2021 18:50


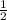 H₂(g) +
H₂(g) + 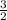 O₂(g) --> COCl₂(g) + 2 H₂O(l) ΔH = 80.5 kJ ----------(3)
O₂(g) --> COCl₂(g) + 2 H₂O(l) ΔH = 80.5 kJ ----------(3)




