
Chemistry, 08.04.2020 19:25, angelaisthebest1700
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total pressure of the gases above an equilibrium mixture if, at equilibrium, PHI = 0.010 × PH2 S?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 18:30, sarahbug56
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
Do you know the correct answer?
For the reaction H2 S(g) + I2 (s) ⇌ S(s) + 2 HI(g) Kp = 1.33×10–5 at 333 K. What will be the total p...
Questions in other subjects:

Mathematics, 09.07.2019 23:30




Computers and Technology, 09.07.2019 23:30

Mathematics, 09.07.2019 23:30


Mathematics, 09.07.2019 23:30

Mathematics, 09.07.2019 23:30


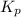
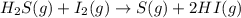
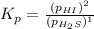
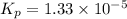
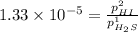
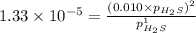
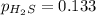
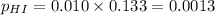
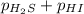 = 0.133+0.0013 = 0.1343 atm
= 0.133+0.0013 = 0.1343 atm




