
Chemistry, 08.04.2020 06:40, cancerbaby209
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If a 3.72 g sample of methane is burned at constant pressure, what will be the value of ∆H? (Hint: Convert the grams of methane to moles. Also make sure your answer has the correct sign for an exothermic process.) Answer in units of kJ.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, motorxr714
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
Do you know the correct answer?
When 1 mol of methane is burned at constant pressure, −890 kJ/mol of energy is released as heat. If...
Questions in other subjects:










Mathematics, 28.10.2019 21:31



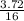 = 0.23moles
= 0.23moles 



