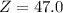Chemistry, 08.04.2020 05:03, munozjosue258
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromium-52 has a percent abundance of 83.79%, chromium-53 has a percent abundance of 9.50%, and chromium-54 has a percent abundance of 2.37%. Based on this information calculate the average atomic mass of chromium. *

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, mauifrifer3986
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1



Chemistry, 22.06.2019 21:50, donttrip10
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state. a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
Do you know the correct answer?
Chromium has four naturally occurring isotopes. Chromium-50 has a percent abundance of 4.35%, chromi...
Questions in other subjects:

Mathematics, 20.05.2021 09:00

Mathematics, 20.05.2021 09:00


Mathematics, 20.05.2021 09:00

Mathematics, 20.05.2021 09:00


Social Studies, 20.05.2021 09:00

Mathematics, 20.05.2021 09:00

Mathematics, 20.05.2021 09:00

Mathematics, 20.05.2021 09:00


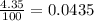
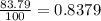
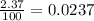
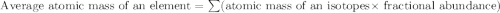
![Z=[(50\times 0.0435)+(52\times 0.8379)+(54\times 0.0237)]](/tpl/images/0589/0146/846f5.png)