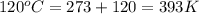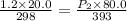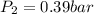Chemistry, 08.04.2020 04:33, guillmar003
A gas inside a piston initially has a pressure of 1.2 bars at temperature of 25 degree C and volume of 20.0 mL. If temperature is increased to 120 deg C and the piston expands to a volume of 80 mL, what is the new pressure

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 23.06.2019 12:00, cooltrey777
How far in advance is weather forecasting most accurate
Answers: 2
Do you know the correct answer?
A gas inside a piston initially has a pressure of 1.2 bars at temperature of 25 degree C and volume...
Questions in other subjects:

Physics, 20.09.2019 15:50


Mathematics, 20.09.2019 15:50


Business, 20.09.2019 15:50




Mathematics, 20.09.2019 15:50


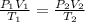
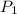 = initial pressure of gas = 1.2 bar
= initial pressure of gas = 1.2 bar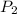 = final pressure of gas = ?
= final pressure of gas = ?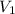 = initial volume of gas = 20.0 ml
= initial volume of gas = 20.0 ml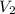 = final volume of gas = 80.0 ml
= final volume of gas = 80.0 ml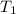 = initial temperature of gas =
= initial temperature of gas = 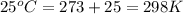
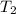 = final temperature of gas =
= final temperature of gas =