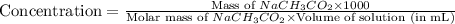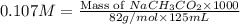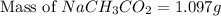Chemistry, 08.04.2020 04:32, carolinasoto
A chemistry graduate student is given 125.mL of a 0.20M acetic acid HCH3CO2 solution. Acetic acid is a weak acid with =Ka×1.810−5. What mass of NaCH3CO2 should the student dissolve in the HCH3CO2 solution to turn it into a buffer with pH =4.47?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
Do you know the correct answer?
A chemistry graduate student is given 125.mL of a 0.20M acetic acid HCH3CO2 solution. Acetic acid is...
Questions in other subjects:


Computers and Technology, 23.03.2021 22:10

Mathematics, 23.03.2021 22:10




Mathematics, 23.03.2021 22:10




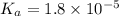
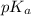 .
.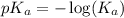
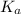 in this expression, we get:
in this expression, we get: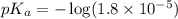
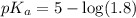
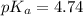
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0588/8914/e961a.png)
![4.47=4.74+\log (\frac{[Salt]}{0.20})](/tpl/images/0588/8914/d9d31.png)
![[Salt]=0.107M](/tpl/images/0588/8914/d66e6.png)