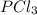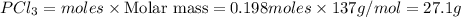For the following reaction, 6.12 grams of phosphorus (P4) are mixed with excess chlorine gas . The reaction yields 21.5 grams of phosphorus trichloride . phosphorus (P4) ( s ) chlorine ( g ) phosphorus trichloride ( l ) What is the theoretical yield of phosphorus trichloride

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 18:50, cj31150631
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1


Chemistry, 23.06.2019 10:20, kyliemorgan8623
El amoniaco y el fluor reaccionan para formar tetrafluoruro de dinitrogeno y fluoruro de hidrogeno. segun la reaccion: nh3 + f2 ⇒ n2f4 + hf si reaccionan 5 gramos de amoniaco y 20 gramos de fuor, ¿cuantos gramos de fluoruro de hidrogeno se producen?
Answers: 2
Do you know the correct answer?
For the following reaction, 6.12 grams of phosphorus (P4) are mixed with excess chlorine gas . The r...
Questions in other subjects:

Arts, 18.05.2021 14:00


English, 18.05.2021 14:00

Biology, 18.05.2021 14:00

History, 18.05.2021 14:00


Mathematics, 18.05.2021 14:00



History, 18.05.2021 14:00


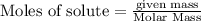
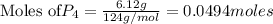
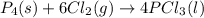
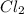 is the excess reagent,
is the excess reagent, 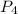 is the limiting reagent as it limits the formation of product.
is the limiting reagent as it limits the formation of product.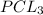
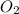 give =
give =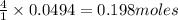 of
of