
Chemistry, 08.04.2020 03:35, rainbowmc6
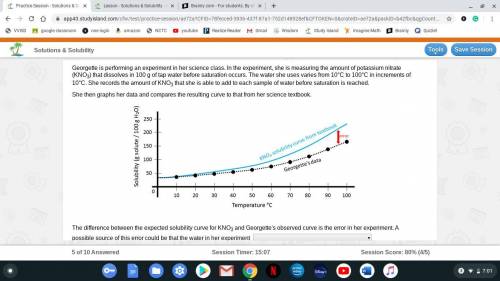
Georgette is performing an experiment in her science class. In the experiment, she is measuring the amount of potassium nitrate (KNO3) that dissolves in 100 g of tap water before saturation occurs. The water she uses varies from 10°C to 100°C in increments of 10°C. She records the amount of KNO3 that she is able to add to each sample of water before saturation is reached.
She then graphs her data and compares the resulting curve to that from her science textbook.
The difference between the expected solubility curve for KNO3 and Georgette's observed curve is the error in her experiment. A possible source of this error could be that the water in her experiment
A. did not have any substances already in it
B. already had substances dissolved in it
C. vaporized at about 40°C
D Solidified at about 40°C

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kaylaamberd
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1


Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
Do you know the correct answer?
Georgette is performing an experiment in her science class. In the experiment, she is measuring the...
Questions in other subjects:






Mathematics, 15.10.2020 08:01

Medicine, 15.10.2020 08:01


Mathematics, 15.10.2020 08:01






