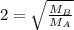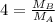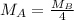At a given temperature and pressure, a sample of Gas A is observed to diffuse twice as fast as a sample of a different gas, B. Based on this: a. The molar mass of A is one fourth that of B b. The molar mass of A is one half that of B c. The molar mass of A is four times that of B d. The molar mass of A is 1.414 times that of B e. The molar mass of A is 0.707 times that of B

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
Do you know the correct answer?
At a given temperature and pressure, a sample of Gas A is observed to diffuse twice as fast as a sam...
Questions in other subjects:

Mathematics, 04.08.2021 22:00

Mathematics, 04.08.2021 22:00


History, 04.08.2021 22:00

Biology, 04.08.2021 22:10

Mathematics, 04.08.2021 22:10

Biology, 04.08.2021 22:10



Mathematics, 04.08.2021 22:10


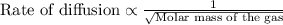
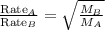
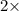 Rate of diffusion of B
Rate of diffusion of B