
Chemistry, 08.04.2020 01:51, habsofarah0
14. In the lab, an experimenter mixes 75.0 g of water (initially at 30oC) with 83.8 g of a solid metal (initially at 600oC). At thermal equilibrium, he measures a final temperature of 50.0oC. What metal did the experimenter probably use

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3

Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
Do you know the correct answer?
14. In the lab, an experimenter mixes 75.0 g of water (initially at 30oC) with 83.8 g of a solid met...
Questions in other subjects:


History, 02.10.2020 15:01



English, 02.10.2020 15:01

Mathematics, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01


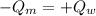
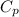

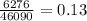 j.g⁻¹.⁰c⁻¹
j.g⁻¹.⁰c⁻¹



