
Chemistry, 08.04.2020 01:33, imeldachavez124
He thermite reaction, in which powdered aluminum reacts with iron(III) oxide, is highly exothermic. 2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(s) Use standard enthalpies of formation to find for the thermite reaction

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1


Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
Do you know the correct answer?
He thermite reaction, in which powdered aluminum reacts with iron(III) oxide, is highly exothermic....
Questions in other subjects:

History, 10.03.2020 23:25



Physics, 10.03.2020 23:25

Mathematics, 10.03.2020 23:25




English, 10.03.2020 23:26

Biology, 10.03.2020 23:26


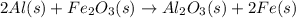 . Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.
. Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.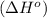 .
.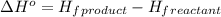
![\Delta H^o=[n_{Fe}\times \Delta H_f^0_{(Fe)}+n_{Al_2O_3}\times \Delta H_f^0_{(Al_2O_3)}]-[n_{Al}\times \Delta H_f^0_(Al)+n_{Fe_2O_3}\times \Delta H_f^0_{(Fe_2O_3)}]](/tpl/images/0588/4390/ac2a9.png)
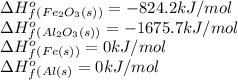
![\Delta H^o_{rxn}=[(2\times 0)+(1\times -1675.5)]-[(2\times 0)+(1\times -824.2)]=-851.5kJ](/tpl/images/0588/4390/65523.png)




