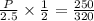Chemistry, 08.04.2020 01:03, kenziepickup
A sample of ideal gas is heated in a 2L vessel at a temperature of 320 Kelvin. the pressure in the vessel is 2.5 atm. What is the new pressure in the vessel if the volume is halved and the temperature is reduced to 250 Kelvin?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3


Chemistry, 23.06.2019 06:00, fjsdfj1284
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2
Do you know the correct answer?
A sample of ideal gas is heated in a 2L vessel at a temperature of 320 Kelvin. the pressure in the v...
Questions in other subjects:





History, 12.02.2021 20:10


Mathematics, 12.02.2021 20:10

Mathematics, 12.02.2021 20:10

Mathematics, 12.02.2021 20:10


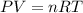
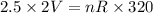 ..............(1)
..............(1)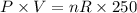 ................(2)
................(2)