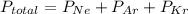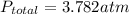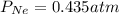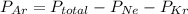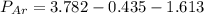Chemistry, 08.04.2020 00:46, isaiahst573
A sealed flask contains Ne, Ar, and Kr gas. If the total pressure in the flask is 3.782 atm, the partial pressure of Ne is 0.435 atm, and the partial pressure of Kr is 1.613 atm, what is the partial pressure of Ar

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
Do you know the correct answer?
A sealed flask contains Ne, Ar, and Kr gas. If the total pressure in the flask is 3.782 atm, the par...
Questions in other subjects:

Mathematics, 21.05.2021 22:10

English, 21.05.2021 22:10

Social Studies, 21.05.2021 22:10

History, 21.05.2021 22:10



Mathematics, 21.05.2021 22:10

English, 21.05.2021 22:10

Arts, 21.05.2021 22:10


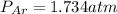 = partial pressure of Ar
= partial pressure of Ar