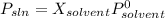Chemistry, 07.04.2020 23:47, mariamsakayanthebest
At a certain temperature, the vapor pressure of pure benzene is atm. A solution was prepared by dissolving g of a nondissociating, nonvolatile solute in g of benzene at that temperature. The vapor pressure of the solution was found to be atm. Assuming that the solution behaves ideally, determine the molar mass of the solute.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
Do you know the correct answer?
At a certain temperature, the vapor pressure of pure benzene is atm. A solution was prepared by di...
Questions in other subjects:




Mathematics, 24.04.2020 21:31




Mathematics, 24.04.2020 21:31