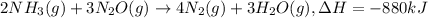When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equation, 880 kJ of energy are evolved. 2NH3(g) + 3N2O(g)4N2(g) + 3H2O(g) Is this reaction endothermic or exothermic? What is the value of q? kJ An error has been detected in your answer. Che

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2
Do you know the correct answer?
When 2 moles of NH3(g) react with N2O(g) to form N2(g) and H2O(g) according to the following equatio...
Questions in other subjects:



History, 22.08.2019 20:00

History, 22.08.2019 20:00



Social Studies, 22.08.2019 20:00

English, 22.08.2019 20:00



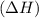 comes out to be negative.
comes out to be negative.