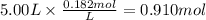The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concentration of N2O5 and at a certain temperature has a rate constant k of 0.0168 s-1. If 2.50 moles of N2O5 were placed in a 5.00 liter container at that temperature, how many moles of N2O5 would remain after 1.00 min?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
Do you know the correct answer?
The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concen...
Questions in other subjects:

Biology, 19.05.2021 19:10

Mathematics, 19.05.2021 19:10

Mathematics, 19.05.2021 19:10



Mathematics, 19.05.2021 19:10


Mathematics, 19.05.2021 19:10

Mathematics, 19.05.2021 19:10

History, 19.05.2021 19:10


![[N_2O_5] = [N_2O_5]_0 \times e^{-k \times t}](/tpl/images/0587/8861/ec7f1.png)
![[N_2O_5]_0](/tpl/images/0587/8861/4a20d.png) : initial concentrationk: rate constantt: time
: initial concentrationk: rate constantt: time![[N_2O_5] = 0.500 M \times e^{-0.0168 s^{-1} \times 60s} = 0.182 M](/tpl/images/0587/8861/ce720.png)