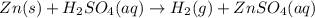Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
Do you know the correct answer?
Solid zinc metal reacts with sulfuric acid to form hydrogen gas and an aqueous solution of zinc (II)...
Questions in other subjects:

Mathematics, 04.11.2020 09:50

Mathematics, 04.11.2020 09:50

Health, 04.11.2020 09:50

Mathematics, 04.11.2020 09:50

History, 04.11.2020 09:50

Mathematics, 04.11.2020 09:50


Mathematics, 04.11.2020 14:00


Mathematics, 04.11.2020 14:00