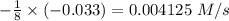Chemistry, 03.12.2019 16:31, madison1284
Consider the reaction: 8h2s(g)+4o2(g)→8h2o(g)+s8(g). δ[h2s]/δt = -0.033m/s. find δ[o2]/δt. δ[h2o]/δt. δ[s8]/δt. find the rate of the reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2



Chemistry, 23.06.2019 01:30, sleimanabir
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
Do you know the correct answer?
Consider the reaction: 8h2s(g)+4o2(g)→8h2o(g)+s8(g). δ[h2s]/δt = -0.033m/s. find δ[o2]/δt. δ[h2o]/δ...
Questions in other subjects:



Mathematics, 10.06.2020 01:57



Social Studies, 10.06.2020 01:57


Mathematics, 10.06.2020 01:57

Mathematics, 10.06.2020 01:57

Mathematics, 10.06.2020 01:57


![\frac{[\Delta O_2]}{\Delta t} = -0.0165\ M](/tpl/images/0401/1414/d412a.png)
![\frac{[\Delta H_2O]}{\Delta t}= 0.033\ M/s](/tpl/images/0401/1414/ccd80.png)
![\frac{[\Delta S_8]}{\Delta t} = 0.004125\ M/s](/tpl/images/0401/1414/d55d1.png)
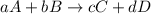
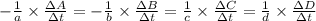
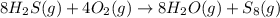
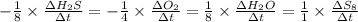
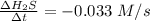
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =-\frac{1}{4}\times \frac{[\Delta O_2]}{\Delta t}](/tpl/images/0401/1414/fd6a9.png)
![-\frac{1}{8}\times (-0.33) =-\frac{1}{4}\times \frac{[\Delta O_2]}{\Delta t}](/tpl/images/0401/1414/02deb.png)
![-\frac{[\Delta O_2]}{\Delta t} = \frac{4}{8} \times (0.033) = 0.0165\ M](/tpl/images/0401/1414/323db.png)
![\frac{[\Delta O_2]}{\Delta t} = -0.0165\ M/s](/tpl/images/0401/1414/d516e.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =\frac{1}{8}\times \frac{[\Delta H_2O]}{\Delta t}](/tpl/images/0401/1414/d13b5.png)
![\frac{[\Delta H_2O]}{\Delta t}=-\frac{8}{8}\times \frac{[\Delta H_2S]}{\Delta t}](/tpl/images/0401/1414/4e162.png)
![\frac{[\Delta H_2O]}{\Delta t}=-\frac{8}{8}\times (-0.033)](/tpl/images/0401/1414/b29cf.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =\frac{1}{1}\times \frac{[\Delta S_8]}{\Delta t}](/tpl/images/0401/1414/1cad1.png)
![\frac{[\Delta S_8]}{\Delta t}=-\frac{1}{8}\times (-0.033)=0.004125\ M/s](/tpl/images/0401/1414/4b7e9.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t}](/tpl/images/0401/1414/78436.png)