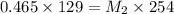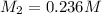Chemistry, 07.04.2020 23:01, iamcuriousdelip08rmf
A 75.0 mL aliquot of a 1.60 M solution is diluted to a total volume of 258 mL. A 129 mL portion of that solution is diluted by adding 125 mL of water. What is the final concentration? Assume the volumes are additive.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, maevemboucher78
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2


Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
Do you know the correct answer?
A 75.0 mL aliquot of a 1.60 M solution is diluted to a total volume of 258 mL. A 129 mL portion of t...
Questions in other subjects:

Physics, 22.01.2021 19:00

Mathematics, 22.01.2021 19:00


Mathematics, 22.01.2021 19:00


Mathematics, 22.01.2021 19:00






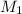 = Molarity of stock solution = 1.60 M
= Molarity of stock solution = 1.60 M = volume of stock solution = 75.0 ml
= volume of stock solution = 75.0 ml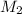 = molaity of diluted solution = ?
= molaity of diluted solution = ?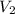 = volume of diluted solution = 258 ml
= volume of diluted solution = 258 ml