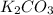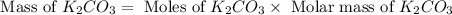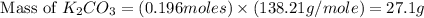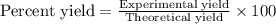Chemistry, 07.04.2020 22:58, timjape3g3z
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27.9 g KO with 29.0 L of CO (at STP). The molar mass of KO = 71.10 g/mol and KCO = 138.21 g/mol. 4 KO(s) + 2 CO(g) → 2 KCO(s) + 3 O(g)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, annan65
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 17:00, brownvester44
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
Determine the theoretical yield and the percent yield if 21.8 g of KCO is produced from reacting 27....
Questions in other subjects:

English, 31.01.2022 15:50


World Languages, 31.01.2022 15:50


Chemistry, 31.01.2022 15:50


Mathematics, 31.01.2022 15:50

Mathematics, 31.01.2022 15:50


Mathematics, 31.01.2022 15:50


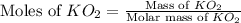
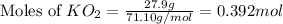
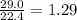 mole of CO₂ gas.
mole of CO₂ gas.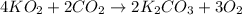
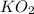 react with 2 mole of
react with 2 mole of 
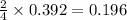 moles of
moles of